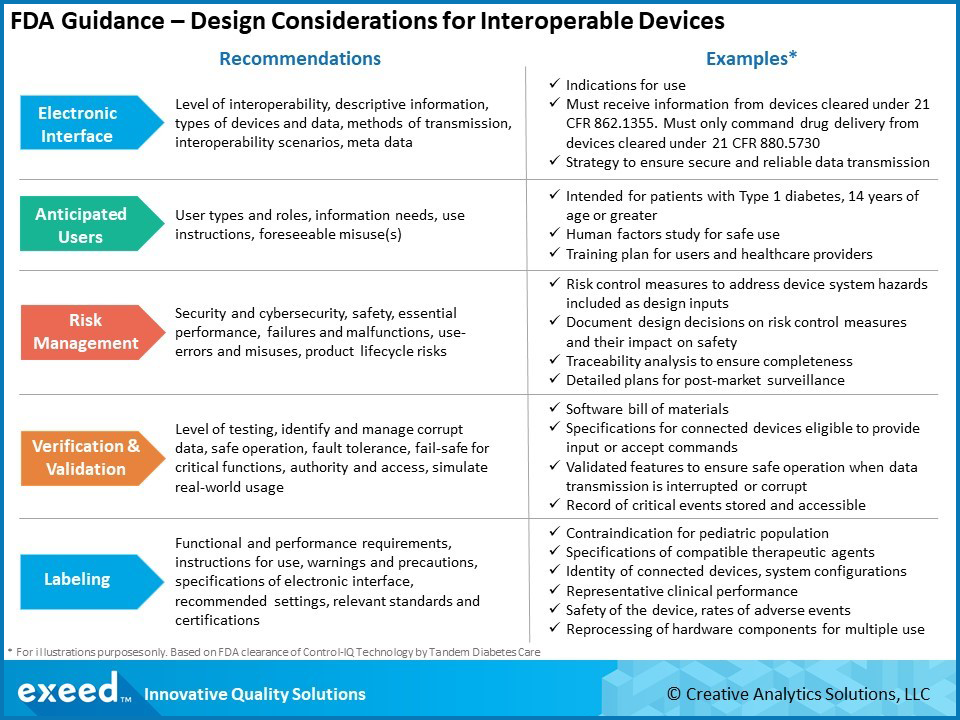
%20A%20Complete%20Guide%20to%20Bringing%20a%20Medical%20Device%20to%20Market.png?width=4250&name=(cover)%20A%20Complete%20Guide%20to%20Bringing%20a%20Medical%20Device%20to%20Market.png)
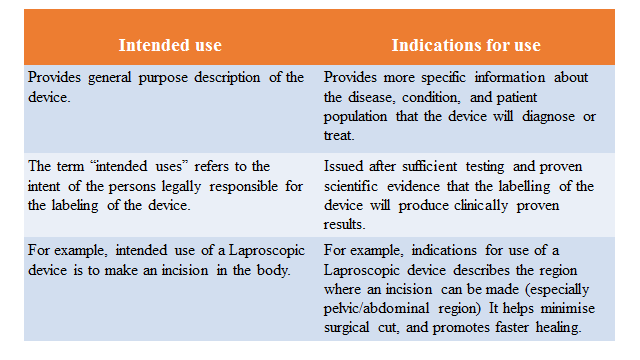
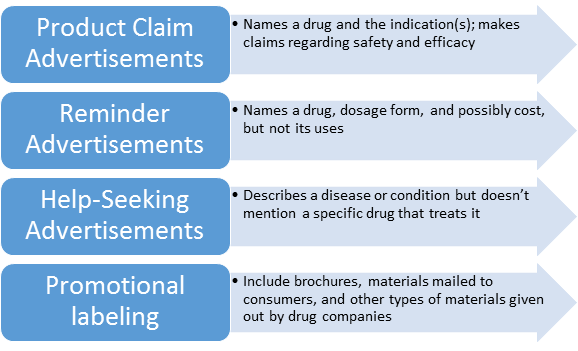
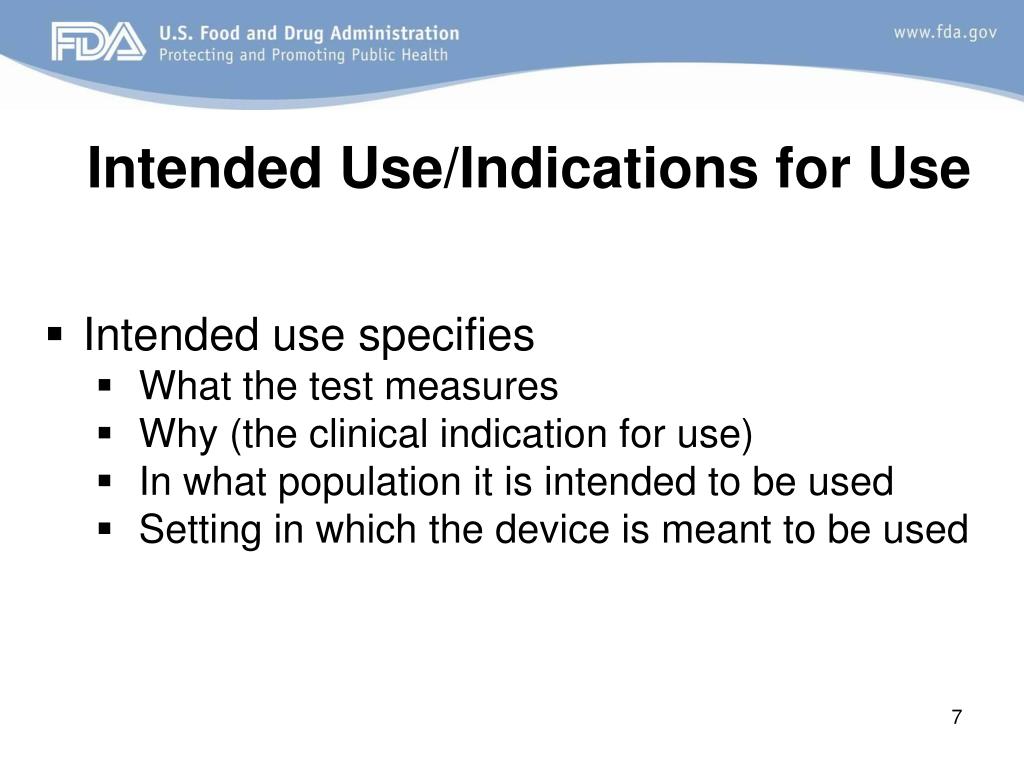
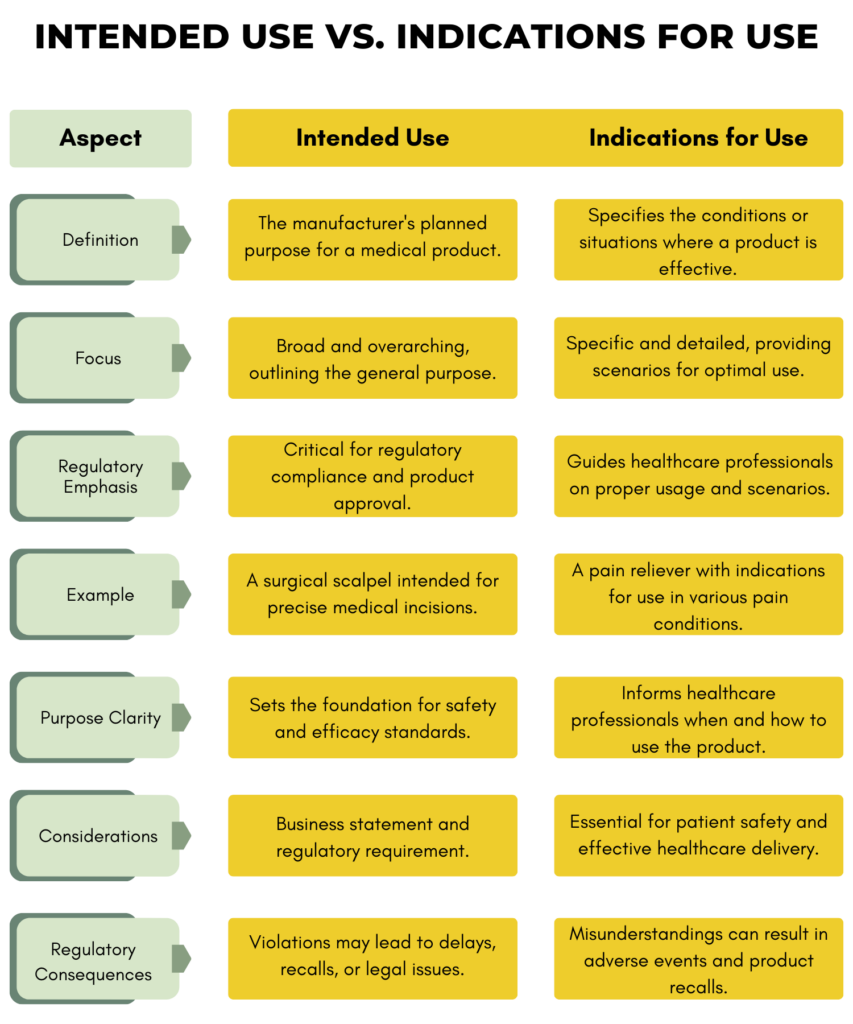
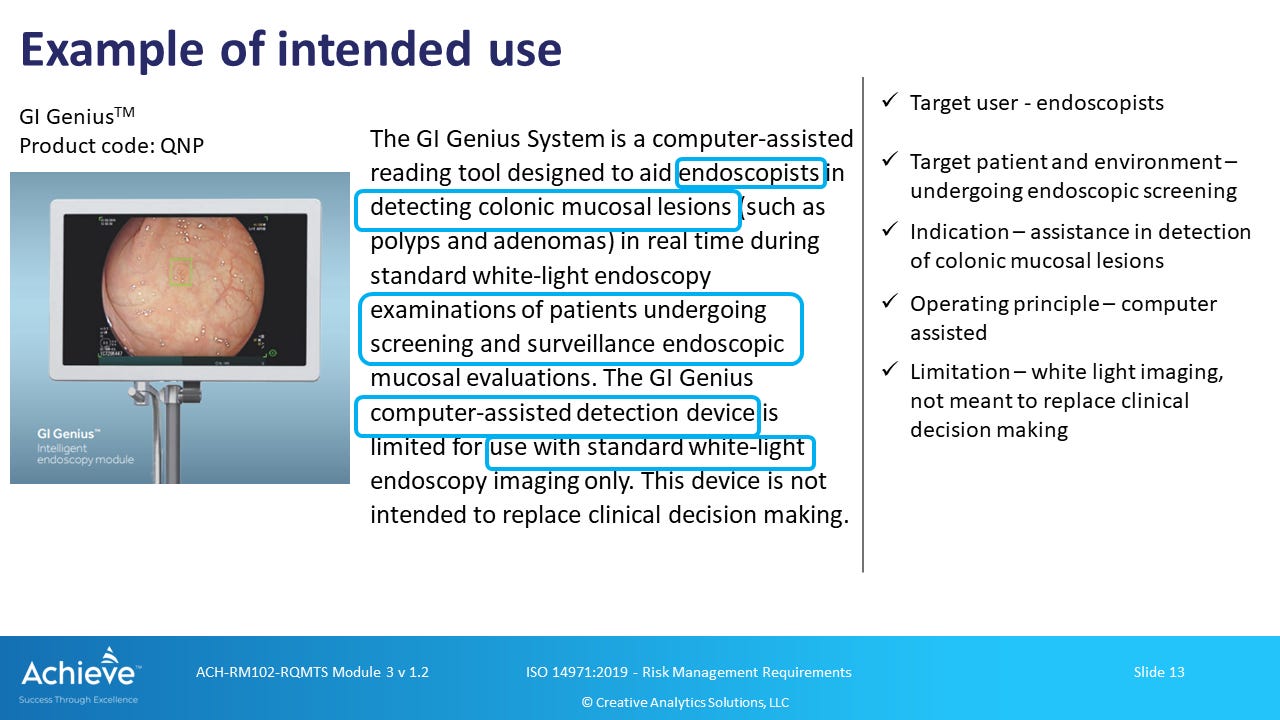
The Difference Between Intended Use and Indications of Use (And Why These Statements Are So Important)

The Difference Between Intended Use and Indications of Use (And Why These Statements Are So Important)

FDA's role in the innovation and evaluation of evolving computer-aided diagnosis (CAD) solutions Kyle J. Myers, Ph.D. Director, Division of Imaging, Diagnostics, - ppt download

How to Prepare for and Make the Most Out of your FDA Pre-Submission: Leverage This Under-Utilized Tool to Help De-Risk your 510(k) | PPT