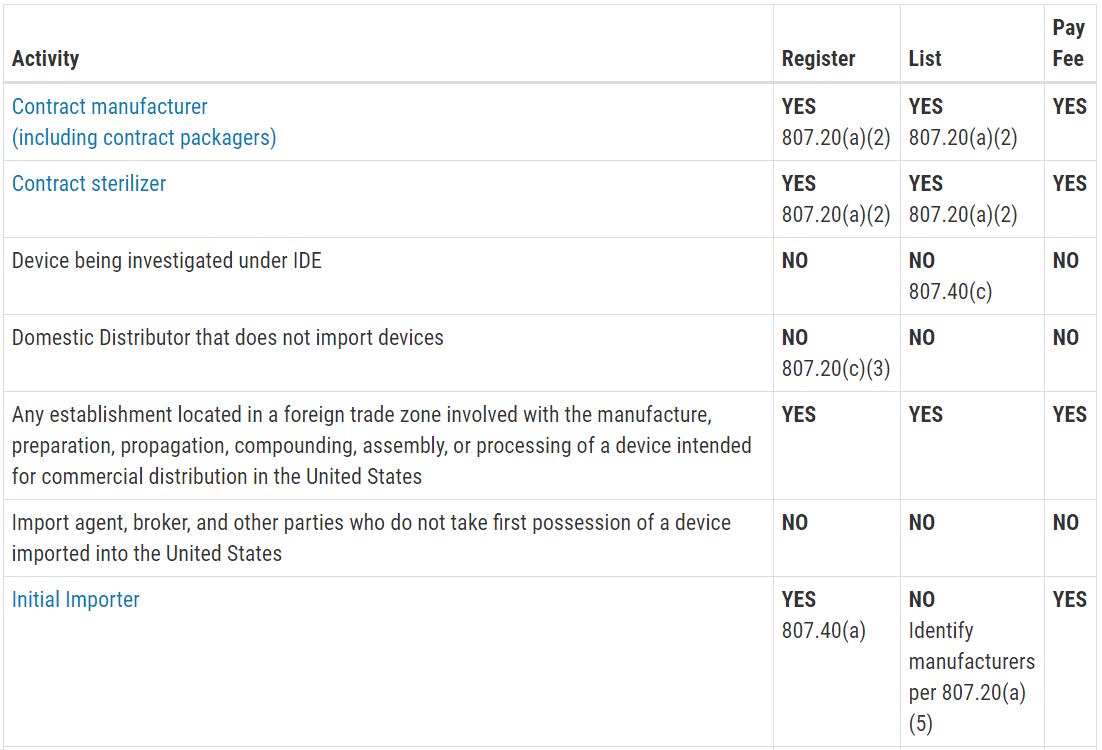
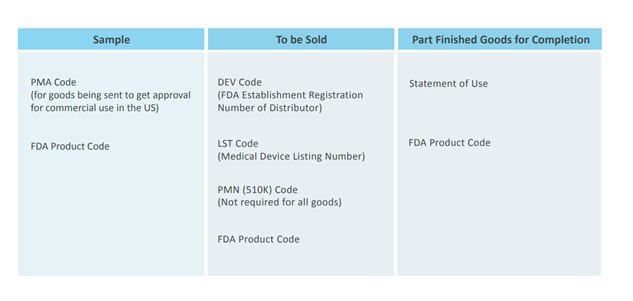
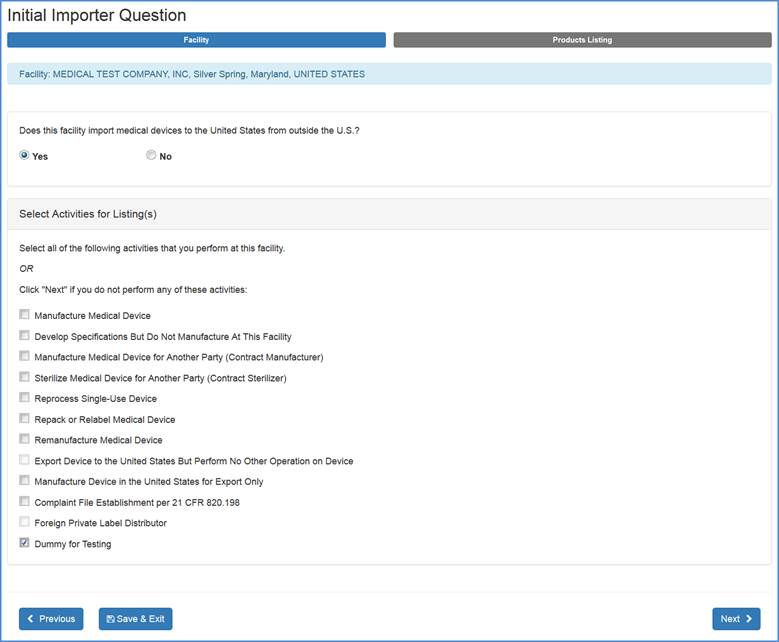
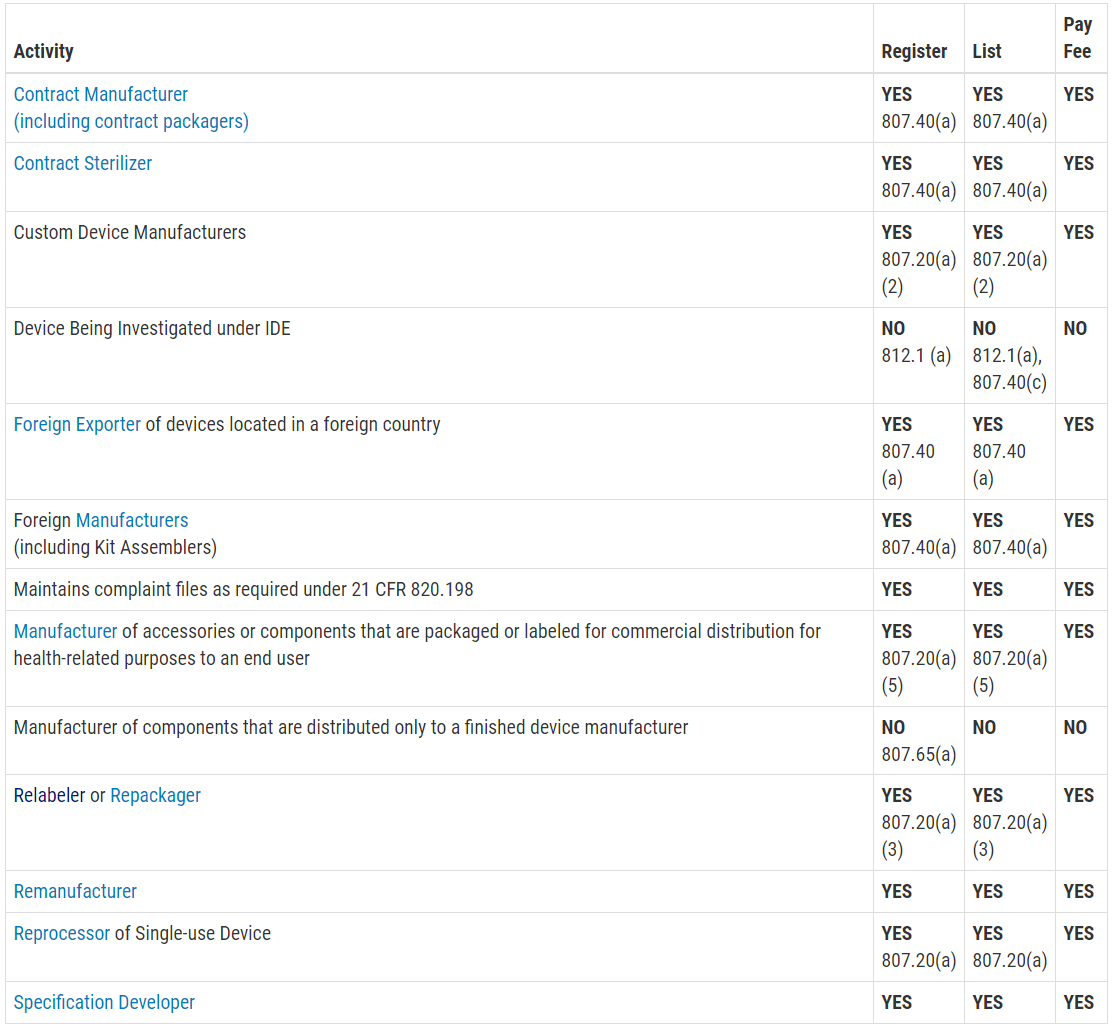
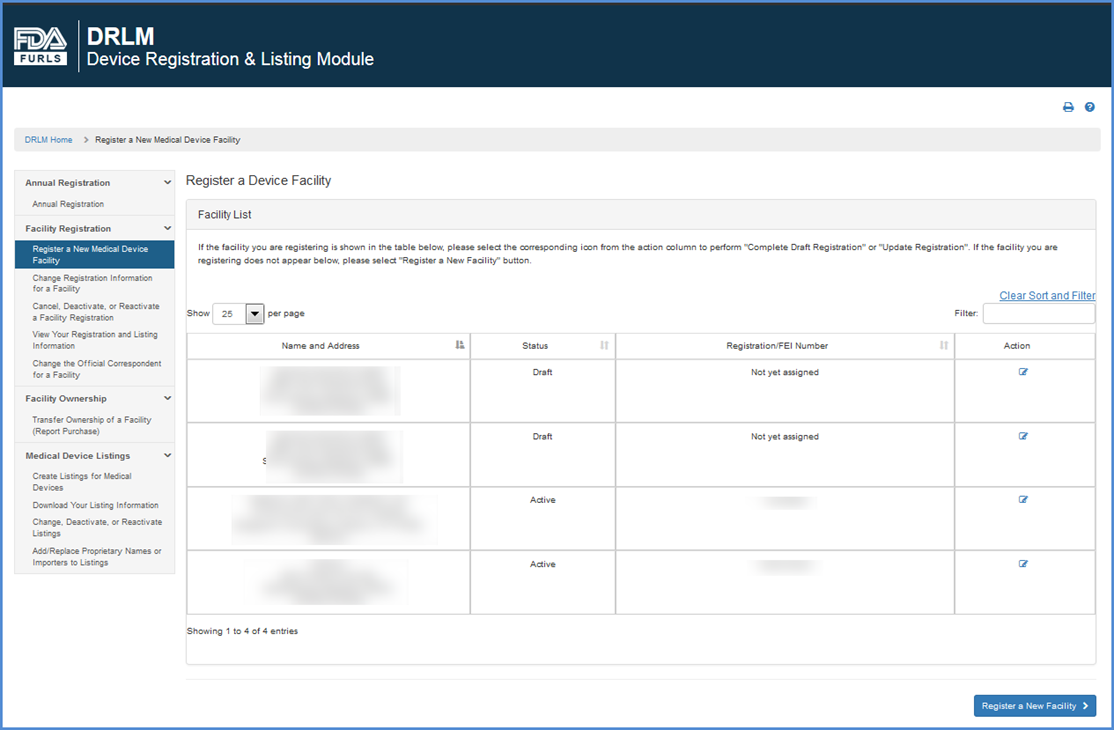
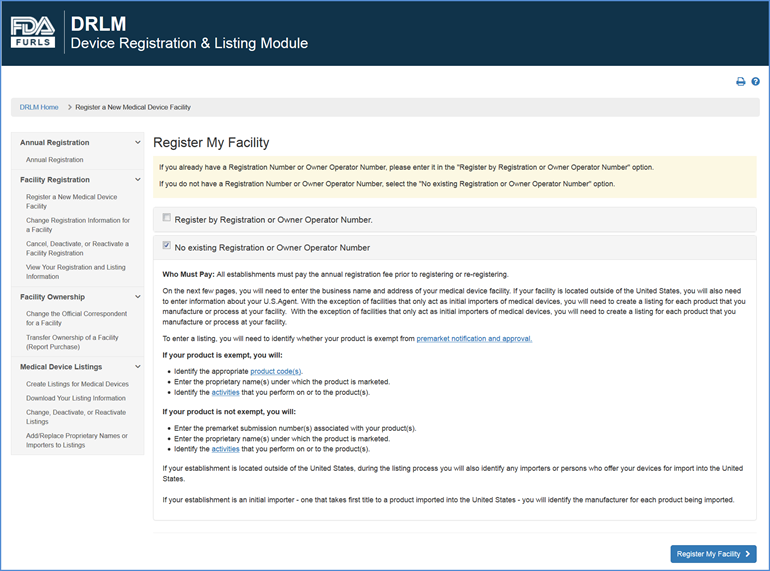
FURLS Device Registration & Listing Initial Registration Instructions for Domestic, First Registration in Account

PPT - What are the FDA Regulatory Requirements for Importing Medical Devices into the U.S.? PowerPoint Presentation - ID:252211