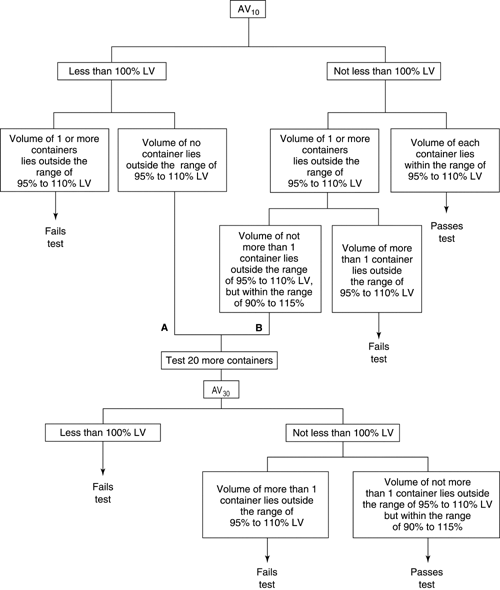
Overfill: How to avoid Volume Loss in Dosage Products | Ahmed Ehab Salah posted on the topic | LinkedIn
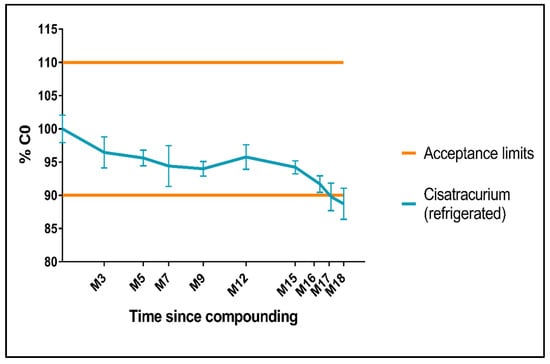
Pharmaceutics | Free Full-Text | Cistracurium Besylate 10 mg/mL Solution Compounded in a Hospital Pharmacy to Prevent Drug Shortages: A Stability Study Involving Four Degradation Products

Impact of Closed System Transfer Device (CSTD) Handling Procedure for Low-Transfer-Volume Dose Preparation of Biologic Drug Products - ScienceDirect

Evaluation of Different Quality-Relevant Aspects of Closed System Transfer Devices (CSTDs) | Pharmaceutical Research