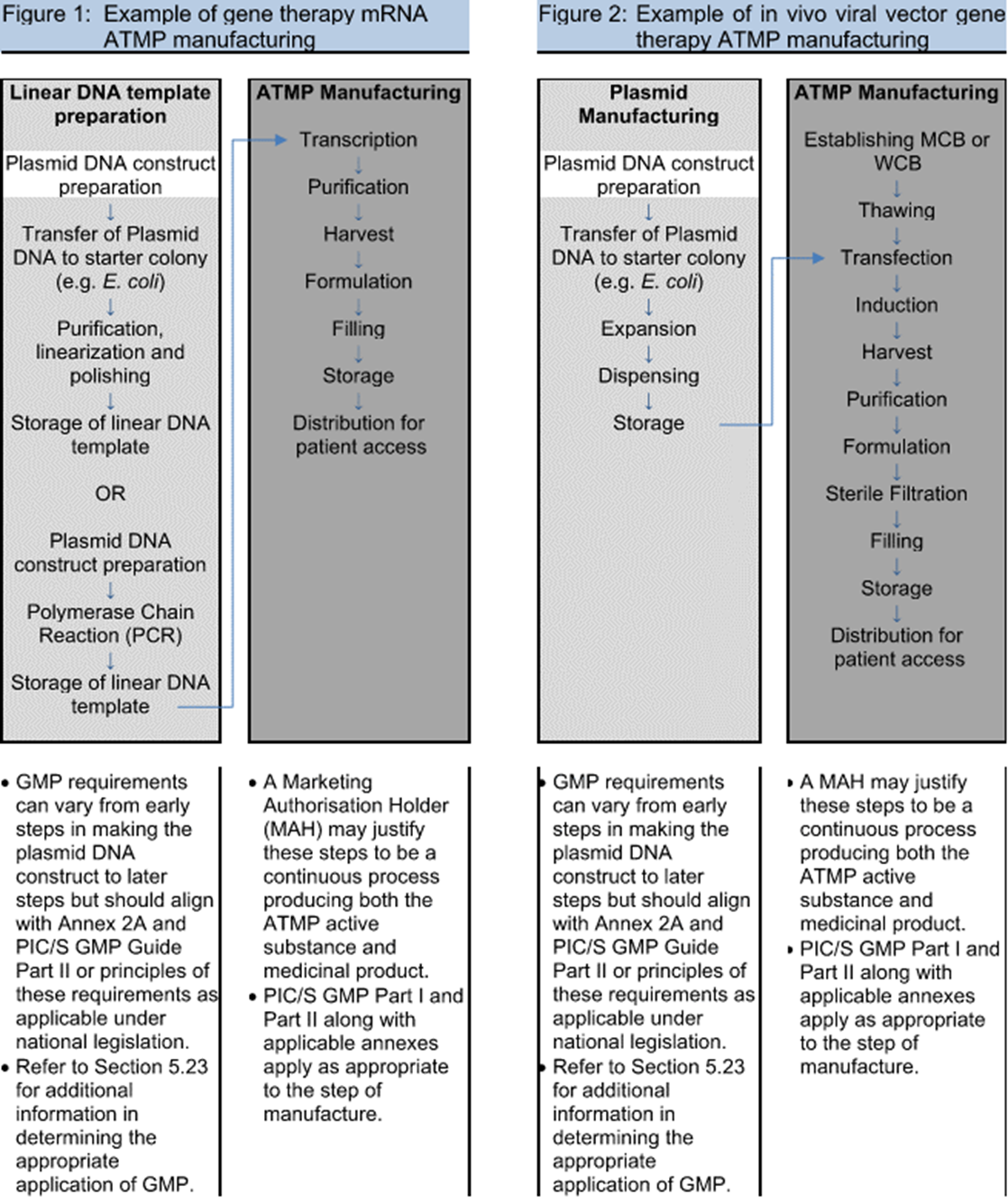
Eudralex Volume 4 Annex 1 – Room for Improvement? - Media Center - AUSTAR Connecting Extraordinary Ideas

EU Commission starts today Consultation on Annex 21 "Importation of Medicinal Products" (EudraLex Vol 4)
New EU GMP Annex 15 Revision published – Valid as of 1 October 2015 – DRUG REGULATORY AFFAIRS INTERNATIONAL